Chapter 7
Marietta Blau on the Margins of Nuclear and Particle Physics
1With the end of the Second World War, the first attempt to bring the scintillation counter back into nuclear research coincided with the passage from what Galison has described as the image to the logic tradition in physics. He argues, "What transformed the scintillator's flash and Cerenkov's glow into basic building blocks of the logic tradition was the electronic revolution begun during the war. When attached to the new high-gain photomultiplier tubes and strung into the array of amplifiers, pulse-height analyzers, and scalers that emerged from the Rad Lab and Los Alamos, then and only then did the scintillator and Cerenkov radiation became part of the material culture of postwar physics."1 Interestingly enough, one of the first to suggest the use of a photomultiplier in combination with the scintillation counter was Blau. Nurtured in the material culture of the Radium Institute before the war, she sought possibilities for professional existence in saving the scintillation counter. It was through this instrument that Blau mingled the competing prewar and postwar cultures in physics research.
From Experienced Experimenter to Industrial Designer
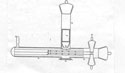
2Although Blau found herself "at the periphery of the American research establishment," as Arnold Perlmutter has argued, it still led her "to an explosion of creative activity."2 Her first position in the United States was at the physics department of the International Rare Metals Refinery, Inc, a corporation located in Mount Kisco, New York. The company processed pitchblende ore for the African Metals Corp to extract uranium and for the Ministry of Economic Development to recover radium and uranium. Blau teamed up with B. Dreyfus in combining the use of a photomultiplier tube to a scintillation screen for the measurement of alpha ray sources.3 As the references to Kara-Michailova's and Karlik's work show, the driving force in designing the device was Blau. Drawing on her work and that of her colleagues at the Radium Institute in Vienna from more than a decade ago, Blau relied on her past to secure her present.
3In 1933, after abandoning the ordinary scintillation counter, Karlik worked on the determination of alpha particle ranges, utilizing a photoelectric cell while she kept the fluorescent screen as the intact part of the instrument.4 Yet, as Blau explained, Karlik's method was seldom used because of the limited range of measurements of the ordinary photocells and the lack of adequate and constant alpha-standards. "These two inconveniences," Blau continued, "have been remedied recently, thanks to the appearance of the multiplier photo-tube, and of good standards." 5
4The continuity of technology traced from Dagmar Pettersson's version of the scintillation counter in 1923 to Karlik's 1933 counter equipped with a photocell and Blau's 1945 detector endowed with photomultiplier is striking. Karlik replaced the microscope used in the early type of the scintillation counter attached directly to the scintillation screen with a photocell that Blau eventually turned into a photomultiplier.6
5By 1933, the shift from the microscope to the photocell was an attempt to save the scintillation counter by excluding the observer. Instead of the fragile and unreliable human optical system, Karlik introduced a sensitive electric device for the detection of the scintillations. The method was barely noticed, however, not only because of its limitations but mostly due to the fact that the scintillation counter had already failed in the experimental tradition of the institute. The end of the Vienna and Cambridge controversy also brought about the end of the counter. Political upheavals soon followed in the country and greatly affected life at the Radium Institute, particularly by determining specific research directions that did not include the scintillation counter.
6After the end of the war, the second shift, this time from the ordinary photocells to photomultipliers in Blau's experimental practice, was not just a simple replacement between two pieces in an instrument. The transformation was a more profound, conceptual one for both the experimenter and her instrument. From a research-oriented position in the Radium Institute in Vienna, Blau's occupation shifted to industrial physics in the postwar United States. Working in Meyer's institute, Blau was challenged by the scientific community to legitimize her theories and instruments. In her new position, she was struggling for her mere existence. Her new concerns were the "wide range of applications" and the possibilities the photomultiplier scintillation counter offered for "quick industrial measurements."7 It is relevant that around the time Blau was hired at the International Rare Metals Refinery, Inc, the company had shifted to primarily producing radium for commercial and medical use.
7Putting together the photomultiplier with a fluorescent screen and using strong polonium sources, Blau and Dreyfus had in fact described the first electrically modified scintillation counter. In the scientific literature, there was no other previous reference to such a device for the detection of radioactive emissions.8 During the war, Samuel Curran and W. Baker had assembled a detector of alpha particles using a photomultiplier, but their report was classified as part of the Manhattan Project and in collaboration with the Radiation Laboratory in California. Although issued on November 17, 1944, it was not published until February 1948.9 Certainly unaware of the previous detector, Blau and Dreyfus were the first to describe the method in the open literature. Used primarily as a detector of alpha particles, however, the multiplier phototube was not limited to alpha or beta measurements, as the authors argued. "In a later article, we will describe its application to the measurements of strong neutrino sources."10
8Instead of exploring the capacities of her new device, Blau was forced to shift her research in another direction. Working for competitive industrial corporations in the 1940s was not quite the same as conducting research in the welcoming atmosphere of the Radium Institute in the early 1920s. More precisely, the corporations that Blau worked for from 1944 to 1948 were deeply involved in the manufacture of nuclear weapons, the commerce of uranium and radium, and the industrial uses of radium.11
9Under the pressure of producing industrial devices, Blau never published her promised work on the measurements of neutrino sources. Her next article, written in 1946 and co-authored by I. Feuer, was on the production of radioactive light sources.12 In the beginning of 1948, Blau moved to the Gibbs Manufacturing and Research Corporation and with R. Carlin, she published on the industrial applications of radioactivity. A number of radioactive devices serving as resistors, electrostatic voltmeters, leveling systems, and micrometers took up Blau's creative time. It is not by chance that they were advertised as "representative examples of the forerunners of a wide range of industrial applications."13
10In her effort to find an appropriate research position, Blau moved again within the next few months, this time to the Canadian Radium and Uranium Corporation (CRUC) as head of the research laboratory.14 Her research experience at Holzknecht's Radiological Institute in Vienna in the early 1920s was now put to use. Carrying over her knowledge of medical physics to the Radium Corporation, Blau designed a photomultiplier scintillation counter for medical use. In a paper published in 1948 and co-authored with J. Smith, she argued that "with the increased availability of radioactive isotopes for medical, biological and industrial research, the problem of suitable instrumentation and consequently units of measurement has presented itself. There is the need for a practical and rugged instrument for routine measurements covering a wide unit in which to express beta radiation."15 Designed for "persons not very familiar with radioactive measurements," Blau's scintillation counter was a convenient and practical instrument for wide use in hospitals and medical laboratories. Despite the fact that she was the first to design and suggest medical applications of the photomultiplier scintillation counter, Blau remained a peripheral figure, trapped in the competitive world of industrial physics.
11Just one year earlier, the efforts to design scintillation counters by replacing the human agent with a reliable and efficient photomultiplier were already at their peak. J. Coltman and Fitz-Hugh Marschall from the Westinghouse Research Laboratories described a photomultiplier scintillation counter for detecting and measuring alpha, beta and gamma rays, and high-energy electrons and neutrons. Kuan Han Sun from the same lab proceeded Blau in extending the detector to neutron measurement.16
12Shortly after, Hartmunt Kallman, from the Kaiser Wilhelm Institute for Physical Research in Berlin, and his student Immanuel Broser greatly advanced the technique by using naphthalene as a fluorescent screen.17 Kallmann's expertise on the photomultiplier scintillation counters served as a passport to the United States. In 1948, he moved to the U.S. Army Signal Corps Laboratory in Belmar, New Jersey, as a research fellow. In 1949, he was appointed director of the Radiation and Solid State Laboratory at New York University's Physics Department.18 The zenith of the photomultiplier era came with the work of Kallmann's student Robert Hofstadter, who left Princeton University to work with Kallmann in New York. As Perlmutter points out, "The development of scintillation counters by Robert Hofstadter was a critical component of his experiments on the scattering of (then) high-energy electrons (600 ev) from heavy protons and heavy nuclei during the 1950s. He received a Nobel Prize in Physics for his work in 1961."19
13By the end of the 1940s, Blau had already lost her chance to play a central role in the uses and applications of the scintillation counter. In 1947, Blau was still not satisfied with her work at the Canadian Radium and Uranium Corporation and made several attempts to find a position at a university or a research institution. In November 1947, both the Agricultural and Mechanical College of Texas and North American Aviation Inc expressed their interest to hire her. Boris Pregel, director of the CRUC, promised her "Although you seem to doubt it, we will be doing scientific work. You know my interest in scientific work and I will not give up easily."20
14Even as Pregel was trying to persuade her to stay at his corporation, Blau received a two-year contract from Columbia University to conduct research on improvements in the use of photographic emulsions for the study of high-speed particles. The research was initiated by the newly established Atomic Energy Committee and was carried out at both Columbia and the Brookhaven National Laboratory. Blau's work was supposed to deal directly with the usefulness of the cyclotron which was then being constructed at Brookhaven, but since the accelerator needed about a year to be ready, Blau was assigned to Columbia laboratories.21 Her contract actually began on January 1, 1948.
15But as Blau remained faithful to the experimental tradition of the 1920s and nostalgic for her work at Meyer's Institute, she was unable to continue her research in the new settings. From the cozy Radium Institute to the impersonal thirteenth floor of the Pupin Physics Laboratories of Columbia, Blau had gone a long way. When she finally moved to Brookhaven two years later, she had only restricted access to the high-energy physics facilities and found herself removed from any cooperating group. Once again, Einstein took the initiative to help her. In a letter of January 5, 1954, he reminded Samuel Goudsmit, director of the lab that "It is well known that Marietta Blau has shown really original achievements. However, it would be very unfortunate if such a personality would be condemned to inactivity due to the shortage of scientific tools. . . Of course, Marietta Blau does not know about this letter."22 Goudsmit's response left no doubt that the days of independent work were already gone:
The difficulties encountered by Dr. Marietta Blau can easily be formulated but are hard to solve. Physics has changed so drastically from the days of simple experimentation that group work has become an unfortunate necessity. Miss Blau's temperament is not adapted to the type of regimentation which occurs nowadays when only intense cooperations make it possible to obtain meager results from a tremendously expensive piece of apparatus.23
16It was at that point that Blau was marginalized in the world of science. In contrast, male scientists such as Kallmann, Broser, and Hofstadter acquired privileged positions in prestigious research universities and centers instead of industrial laboratories. For Kallmann and his research students, the shift from small to big science and the growth of large-scale research came smoothly, giving them a chance to adjust to the new status of physics research. With the war over, the scintillation counter became a powerful instrument in a number of different disciplines. The new technology was essential for high-energy physics research, weapons control and guidance systems, civilian mass communication, and medicine.24 In 1949, portable scintillation counters were developed for fieldwork in geology and for the detection of uranium and radium ores.25 As Hans-Jörg Rheinberger has shown, the production of the liquid scintillation counter in 1953 "opened new epistemic dimensions for radioactive experimentation in biology and medicine."26 In the 1970s, the instrument was finally transformed into a generic technology in molecular biology and medical laboratories. Interestingly, a report issued in 2003 by the Human Resources Development group in Canada concerning medical radiation technologists showed that 77 percent of the personnel who operate scintillation counters and other kinds of radiation detection equipment were women.27
The Nobel Prize and the Culture of Postwar Physics
17On January 8, 1950, while Blau was struggling to survive in the world of high-energy physics, Erwin Schrödinger addressed the Nobel Committee of Physics. "I herewith propose that this year's Nobel Prize for physics be given to Marietta Blau and Hertha Wambacher for experimental work conducted and published in common." Stressing the importance of their method, namely the photographic emulsions technique, he continues, "The method has become of increasing importance in the last years both for studying those nuclear explosions and for revealing details of the nature of ultimate particles and their decay into one another. It has become one of the most important tools for promoting our knowledge of the constitution of matter." As Schrödinger argued, Blau and Wambacher were certainly worthy candidates for the prize as they had priority for using the method and of discovering the first "stars," results of the disintegration process of nuclear particles originated in cosmic radiation.28
18That year, the committee had to evaluate 28 nominees, among them important figures such as Hans Bethe (Nobel Laureate in 1967), Arnold Sommerfeld who had been nominated 81 times between 1901 and 1950 (although he never won), Pyotr Kapitza (Nobel Laureate in 1978), and Cecil Powell. In general, Nobel prizes in the physical sciences are awarded in a three-stage process which involves the committees for physics and chemistry, the relevant section of the academy, and a final meeting of the whole academy. Each nominee is examined in a special report prepared by a committee member.
19Three of the five members of that year's committee belonged to the Siegbahn school of X-ray spectroscopy. The chairman was Manne Siegbahn. He was a Nobel Laureate in physics in 1924 for his research in X-ray spectroscopy and Pettersson's earlier critic. Siegbahn had dominated Swedish physics with his authoritarian style since the 1920s, influencing university appointments and research orientations. Svante Arrhenius referred to him as the "small pope" of Swedish science.29 Siegbahn's former student and chair of experimental physics at Stockholm University, Erik Hulthén, and his successor at Uppsala University, Axel Edvin Lindh, were also members of the committee. The fourth experimentalist was Gustaf Ising, the prominent Swedish physicist who proposed the first accelerator that used time-dependent fields in 1924. Thanks to Rolf Wideroe, Irsing's concept was tested and demonstrated through the design of the first RF linear accelerator (LINAC) in 1927. The fifth member, Ivar Waller, was the only theoretician in the committee and a professor of theoretical physics at Uppsala University. The remaining member was Carl Olof Gudmund Borelius, a professor of physics at the Royal Swedish School of Technology. He was called in as an additional member to participate in discussions and advise on decisions that year. Borelius had been a member of the Royal Swedish Academy of Sciences since 1942.30
20The evaluation of Blau and Wambacher was conducted by Lindh as the most appropriate to provide information on their experimental work and guide the committee members to their final recommendation. His thorough and largely neutral account of their writings indicates that the two women were indeed the first to use the photographic method to demonstrate cosmic radiation and the particle effects of the disintegration. Lindh, however, diminished their achievement by stressing two things: first, the improvements of the photographic plates that the Ilford laboratory had accomplished, and second, the fact that disintegration stars had been previously observed by other physicists such as Carl Anderson and his student Seth Neddermeyer by means of the cloud chamber. Lindh therefore argued that the work of the two Viennese women did not add up to a Nobel Prize.31
21Blau had previously acknowledged the role of the photographic giant Ilford in thickening emulsion layers on the photographic plates to make the entire particle tracks visible. Despite Ilford's help, she insisted that still thicker emulsion layers had to be obtained as the thinness of the plates continued to put geometric constraints on the capture of the particles' paths. In 1932, Blau and Wambacher discovered that pinacryptol yellow sensitizes photographic emulsions to energetic protons. It was not until 1935 that the Ilford laboratories developed independently photographic emulsions sensitive to energetic protons without preliminary immersion in pinacryptol yellow.32 Although important, Ilford's contribution was not enough by itself to ensure Blau's and Wambacher's experimental success.
22Concerning Lindh's second argument, in the summer of 1936, Anderson and Neddermeyer had mounted their cloud chamber on an old flatbed truck and drove it to the top of the Pike's Peak. During their month and a half of experimenting, they took about 10,000 photographs, but, as Maurice Shapiro explained in a later interview to Laurie Brown, in the course of a whole summer of expansions of the cloud chamber, Anderson's and Neddermeyer's results were rather limited. Their photographic plates revealed clusters of tracks but would usually not see the interaction. Whereas the chamber discovered particles and processes only during the brief moments of exposure, the photographic method had the advantage of registering continuously. It was around the same time that Shapiro was advised to look into Blau's and Wambacher's work.
I became aware that this technique [photographic emulsion] had been used with some success in collecting quite impressive accounts of data statistically on the "stars" and that this photographic emulsion technique had the marvelous attribute of being a completely visual technique in that the stars would have the vertex and would be generated somewhere in the middle of this emulsion. Then when the particle emerged, they were all visible.33
23In his 1941 scrupulous review of the photographic emulsion method, Shapiro made it clear that Blau and Wambacher were the first to observe the simultaneous ejection of several particles from a nucleus on plates exposed to cosmic radiation. Two years later, commenting on the history of the method, Cecil Powell also acknowledged that "notably" Blau and Wambacher have employed the method for experiments in nuclear physics.34 So why did Lindh present this two-pronged argument against an award for Blau and Wambacher?
24It is all too easy to argue that Lindh and that year's Nobel committee discriminated against the two female scientists, but one thing is clear. From 1901 to 1950, the Nobel population—those people who were nominated for the prizes in physics and chemistry—was predominantly male with only eight female candidates, of whom just three became prize winners. Lindh's report does not indicate a direct gender bias and Siegbahn's previous opposition to Meitner's Nobel Prize award can only be a fragile indication of systematic gender discrimination.35 But the subtle discrimination against female scientists that occurred after the end of the Second World War and the changing culture of high-energy physics are powerful parts of Blau's and Wambacher's history.
25In a splendid royal ceremony on December 10, 1950, the Nobel Prize was finally awarded to Cecil Powell, the physicist who turned Blau's technique into an "extremely effective aid," in Lindh's words, for the study of mesons. Throughout its report, the Nobel committee seemed intent all along on awarding the prize to someone who was doing follow-up work to the previous year's prize winner, Hideki Yukawa, on the existence of mesons. Powell had been proposed by the largest number of nominators, among them Enrico Fermi. As he had experimentally demonstrated Yukawa's theory, he appeared to be the most obvious candidate. As the committee bluntly put it, "Powell partly developed the photographic method, deployed earlier, into an outstanding, useful tool for the study of nuclear particles and processes and partly by using this method, has made foundational discoveries concerning mesons and their properties. Powell has thereby laid the grounds for a new era in research on mesons."36
26Located at his laboratory at the University of Bristol, Powell utilized Blau's work in Vienna to undertake experiments both in cosmic radiation and, by using the Bristol high-tension generator, to produce a powerful beam of needed particles. Starting in 1938, he expanded his method by dividing the emulsion into sectors and assigning each sector to a different group of scanners, all of whom were female observers. In a wonderfully written account, Galison has demonstrated how Powell turned an old technique of an unsystematic cosmic ray research into a tool for the detailed analysis of accelerator-produced as well as cosmic ray nuclei.37 By 1947, Powell and his collaborators had discovered the existence of primary and secondary mesons, determined their masses, and explored their properties.
27Through the work at Bristol, the reliability of the photographic method increased as the emulsions and the optical equipment for analyzing particle traces were improved. One of the nominators captured the significance of Powell's work: "His special claim to consideration is, in my view, the fact that discoveries of fundamental importance can still be made with the simplest apparatus—in this case, special nuclear emulsions developed under his general direction and microscopes."38
28Although the method was still based on the same simple principle that Blau and Wambacher had put it to use earlier in radioactivity research, Powell had deeply transformed the culture that surrounded its use. By the time he was nominated for the Nobel Prize, Powell was directing a large group of female scanners and experimenters, he had strong university affiliations and powerful industrial connections, and he managed significant material sources. As Galison put it, Powell's lab was "a cottage industry" with its links to chemists, theorists, and scanners.
29In contrast, Blau, the unsuccessful Nobel candidate, tried to continue her emulsion work during the 1940s. However, she never obtained a stable professional affiliation to a university and moved constantly from one industrial site to another, working primarily with the commercial uses of radium. Even when working in the field of photographic emulsions at the Brookhaven Laboratory, Blau did most of the scanning herself despite the common practice to have the trained scanners, usually women, perform the actual scanning of bubble chamber images.39 Throughout the 1940s and 1950s, she remained largely peripheral, working for industrial firms and having only restricted access to high-energy physics machinery.
30A study of the Nobel nominations in physics from 1901 to 1950 reveals an interesting trend that sheds light on Blau's case. The vast majority of the nominees—67 percent—were based in university teaching departments and laboratories. Only 10 percent worked in institutes of technology, and hardly anyone was an industrial physicist.40 Thus, Blau's possibilities of obtaining an award were closely interrelated to a number of more nebulous considerations such as the committee's stance toward university rather than industrial physics research or a cutting edge, specific discovery rather than a life's work. These considerations were closely interrelated to women's limited possibilities for research and experimentation during the 1940s and 1950s.
The End: Blau's Return to Vienna
31In 1955, Blau moved once again from the Brookhaven Laboratory this time to the University of Miami. As Harry Robertson, the head of the physics department explained to Blau, they were in need of teachers for graduate and undergraduate courses who can also carry on research programs. They did not possess, however, any facilities for high-energy nuclear physics. Ingenious as usual and always having to put up with practical constraints in her experimental work, Blau proposed research on "elementary particles and hyperfragments with nuclear emulsions, exposed to the machines in Brookhaven and Berkeley. The work could be done in collaboration with students and the exposed emulsions could be used as tools in teaching nuclear physics." This plan would only require, as she put it, "several good microscopes and one scattering microscope."
32A year later, in collaboration with younger colleagues at the department, Blau applied for a research grant to the National Science Foundation, a project that was actually funded by the Air Force Office of Scientific Research. Setting up from scratch the missing facilities for high-energy physics research at her department, Blau was assured of a three-year grant for the study of photographic emulsions exposed to the Bevatron at the University of California and the Cosmotron at Brookhaven. She was able to remain active in the world of emulsions and the Air Force satisfied its intense interest in what hazards lie in the space their jets and missiles were soon going to penetrate. 41
33But times had changed not only concerning possibilities for experimental work but also concerning the politics of science. On May 2, 1957, Morton Miller, the dean of the University of Miami, addressed the U.S. Department of State at Blau's request with an unusual question. Blau had been recently nominated for the Leibnity Medallion by the Academy of Sciences in Berlin, honoring her achievements in physics. Once again, Schrödinger had placed the nomination,42 but the Second World War had not only displaced most of the prominent European scientists such as Blau, it had also created a deep gulf of animosity between East and West.
34The Academy of Sciences, as Miller argued, resided in East Germany in the Soviet sector of Berlin. Schrödinger was also associated with the academy and obviously these facts could pose serious issues in accepting an award from a communist scientific institution. Working with a research grant from the U.S. Air Force, Blau also did not want to risk her position. The immediate answer from the Department of State was definitely expected:
This government does not, as you know, recognize the regime which controls the Soviet zone of Germany. By the same token, the Department of State deprecates any action which tends to clothe this regime or any of its agencies with legality or respectability. Acceptance of a medal from the East German Academy of Sciences would, it seems to us, constitute such an action. In addition, it is quite likely that Dr. Blau's name would be misused by the East Germans in a way which might be embarrassing to her. There is no genuine scholarship in the Soviet-controlled part of Germany, and it therefore seems beyond doubt that the offer is being made to her solely for propaganda purposes.43
35In its boldness, the paragraph reflects the ways postwar science was intertwined with politics. Blau, caught in the shift from small to big science and stood at the nodal point between prewar internationalism and postwar nationalism concerning scientific culture. The era in which the Curies had refused to patent their discovery of radium and polonium were gone. When Blau declined the honor, she was already 63 years old. Because of her prolonged exposure to radioactivity, she started to develop cataracts, requiring an operation. At the end of her life, facing financial and health problems, Vienna seemed the most suitable destination. In March 1960, she requested a release from her university contract. Having no retirement rights or health insurance, she left Miami at the end of May 1960.44
36In Vienna, a number of old colleagues tried to gather funds for her, and Schrödinger put her up for the Schrödinger Prize which she received in 1962. Poor, disconnected from any major scientific network, and bitter with several members of the Radium Institute for accepting the Nazi Stetter as one of the heads of the Physics Institute after the end of the war, Blau distanced herself from serious research and from old friends such as Karlik. She died on January 27, 1970, at Lainz hospital, lonely and unknown to the international physics community.
A Considerable Shift in Trafficking Materials
37In writing this account of the men and women who worked at Vienna Radium Institute, I have been trying to emphasize the history of radium. This book has focused on a major trafficking material and the gendered experimental practices that it generated in the early twentieth century. Among the many sites in which radium appeared—scientific, medical, technological, and industrial—I have focused on Vienna's physics and chemistry laboratories. I have paid attention to the city's medical institutions and to those other sites where scientists—mainly women—moved, carrying radioactive materials. I have been concerned with the controversies that radium spawned in physics and chemistry over its properties and with the conflicts that occurred when it was connected to the atomic structure. Briefly, I have argued that by being a trafficking material, radium brought together men and women in scientific laboratories; enforced novel laboratory designs; constituted new scientific networks among chemists, physicists and physicians; created opportunities for professional careers and unprecedented collaborations; dictated the design of new techniques and instruments; imposed new experimental practices; and changed laboratory culture. It prevailed in medicine and forced physicians to become familiar with radium technologies, share their power with physicists within their hospitals, and collaborate with them in order to produce new medical instruments and standardized radium doses for cancer treatments.
38In its early days, radium built a whole new domain of knowledge at the intersection of physics and chemistry and at crossroads of the laboratory, the lecture hall, the workshop, and industrial and commercial sites. This scientific object became a valuable commodity with a life of its own, inundating the shelves of beauty shops and apothecaries. Radium mattered in the public's eyes both through commercial advertisements and physics laws. On the one hand, the industry created a powerful market for the new material with the surprising properties and on the other hand, scientists built careers and networks working with radium as a therapeutic agent and a means for probing nature.
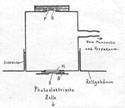
39Radium's rapid expansion in the scientific world relied on a complex system of chemistry and physics technologies, on standardization techniques, and on the institutionalization of the new field of radioactivity. As we saw in the case of Vienna, designing a new institute to host radium research meant designing according to the new experimental culture of radioactivity, taking into account the interdisciplinary nature of the field and the gendered experiences of those working in the institute's laboratories. The architecture of the Radium Institute materialized the identities that the physicists working on radioactivity wished to acquire. Built as a separate building from the physics and chemistry institutes, the Radium Institute legitimized research on radioelements as a scientific specialty, which was still dependent on physics and chemistry.
40The new building provided and stabilized professional identities for those physicists whose new disciplinary identities were at risk of blending with well-established ones. At the same time, the internal spatial arrangements of the institute reflected the ways through which the architecture of the laboratory acknowledged that physicists were gendered. The separate sanitary installations for men and women indicated the existence of both in the institute. Moreover, by having a work bench for themselves, women gained a sense of belonging. They were no longer transients in the field. Here, the city played an additional role.
41As a context for intellectual work, Vienna, and especially the Mediziner-Viertel, was linked to physicists' self-images and their interdisciplinary practices. The urban reconstruction of the city becomes sociologically interesting for it provides the space to study the social and political negotiations that shaped the concrete realization of scientific institutions. Implementation of the decision to move from the ramshackle Physics Institute in Türkenstrasse to the new natural-science quarter across from the Josephinum took physicists more than three decades. Moreover, it involved endless negotiations on the nature of their discipline, the exact location of their institutes in relation to the exchange of radioactive materials, their professional practices, and the identities they wanted to portray through the architecture of their buildings.
42The location of the institute at the center of the cultural and epistemic life of Vienna worked especially in women's favor. In one sense, the few women in the early-twentieth-century science became visible in the relatively small scientific community of Vienna. Several times a day, women crossed Währingerstrasse in order to attend classes and participate in laboratory courses. The face-to-face interactions in the Mediziner-Viertel affected their social lives and contributed to their visibility in the community. For example, it was not hard for Meitner to recall that Horovitz had been a chemistry student when Hönigschmid asked her to suggest a possible collaborator to him.
43Although few in absolute numbers, the women who entered the Radium Institute during its first decade were surprisingly numerous considering that the University of Vienna had started admitting them less than two decades before. In another sense, the crossing of Währingerstrasse facilitated the crossing of disciplinary boundaries as well. From physics to medicine and chemistry to physics, women transferred their knowledge among disciplines, shifting and shaping the boundaries of radioactivity. More importantly, they literally transferred radium preparations, needles, and tubes from the physics laboratory to the clinic.
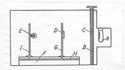
44In the interwar period, radium inaugurated significant changes in laboratory organization. By welcoming different researchers with diverse research projects, the laboratory space had been reassigned and the material basis for experimental work had changed dramatically. During the 1920s, scintillation counters, Shimizu-Wilson ray track apparatus, photographic emulsions, and powerful microscopes become part of the material culture of the Radium Institute. After Pettersson's arrival in Vienna, polonium sources, spent radon needles from the neighboring hospitals, and photographic plates lay around the workbenches and occupied the interest of the experimenters.
45The patterns of experimentation were transformed as well. Although several researchers worked closely together before the 1920s, those groups were limited to two people. For instance, Hönigschmid teamed up with Horovitz, Ludwig Flamm worked with Mache, and Hess with Lawson. With Pettersson's arrival, the groups grew appreciably and complicated the patterns of collaboration. "Our particular kind of work," Pettersson noted in 1928, "requires the close and continued collaboration of at least a dozen highly specialized people."45 At the same time, Przibram gathered around him a number of students and occasionally attracted experienced researchers from Pettersson's team. Karlik and Kara-Michailova, for example, often shifted to Przibram's research projects and co-authored papers with him.
46Experimental scientists such as the Viennese "radioactivists," continued to deal with material units and constantly needing radium in their daily bench work. Even in the midst of the Cambridge-Vienna controversy, Pettersson thought of asking Rutherford for the capillary-spent radon tubes from hospitals used in the preparation of radium and polonium that were useless to the affluent British researchers but essential for the work in Vienna. In addition, Przibram made it clear to the Rockefeller Foundation that funding for an assistant was less important than money "for new apparatus, for replacement of breakage, for liquid air (or for a liquid air machine which could be a source of income to them through their ability to sell it to physicists and chemists, all of whom need air and now have to purchase it from a concern on the outskirts of Vienna)."46 Thus, materials are not fixed entities, but they mean what they mean as far as they can be placed in a local experimental context and used in specific experimental practices.
47For the period preceding the outbreak of the Second World War, radium was also connected to several attempts to exercise political power. The plan of Meyer and the directors of the Vivarium to create a centralized station for controlling radium supplies for the whole country is such an example. In the midst of the 1930s political unrest, controlling radium supplies meant securing research autonomy. In a similar vein, radium transformed medical practices and the organization of work in the clinic. The application of radium therapy replaced that of radiotherapy or rontgentherapy (the use of x-rays in medical treatments). This transformation was particularly evident in Tandler's attempts to bring in radium therapy to municipal hospitals. His venture introduced reorganization of medical work while creating vocational opportunities especially for the female scientists who, unable to foster academic careers, looked for other prospects.
48Finally, in configuring and reconfiguring trafficking materials, scientists were forced to shape new experimental practices, which were often heavily gendered. Work on radium became connected with the gendering of experimental skills as in the case of the Viennese women who designed and handled the scintillation counter. For them, radium also worked as a vehicle for crossing disciplinary boundaries and moving from physics to oceanography. Trading their expertise to prepare radium sources and analyze radium samples became another way for women to finance scientific research and professionally survive when grant money ended and political unrest arose.
49Nonetheless, since the mid-1930s, changes in nuclear physics had repercussions both on materials and instruments and on experimenters and their practices. The development of new technologies such as the particle accelerators and experiments for the production of artificial radioelements or radioisotopes with these new machines forced radium into disuse. Likewise, its decline pushed many of those who knew how to prepare it and handle it into despair, especially the women. Immediately after the Second World War, women such as Blau who had been nurtured in a small-scale laboratory found themselves far from cutting-edge research, unable to adapt to highly hierarchical research groups and having limited access to experimental machines. As their expertise in experimenting with radium lost significance, they lost their ability to survive in the field as well. The shift from radium to radioisotopes marked an important shift not only in the kind of trafficking materials but also in the type of experimental practices each of them embodied.
50Radioisotopes are the product of irradiation in the cores of nuclear reactors. If it is suitably adapted, any reactor could irradiate isotopes. Although production of radioisotopes had started in the prewar period, it did not become widespread until after the Second World War, dictating a new experimental tradition. Making use of large instruments such as the cyclotron, physicists, chemists, and biologists started to manufacture material substances, generating new markets and practices. In contrast to radium, radioisotopes were relatively cheap and easy to make. They were used in radiodiagnostics, new cancer treatments, in thyroid conditions, in nuclear medicine as tracer material, and in biology for investigating organic metabolisms and visualizing elusive molecular processes.
51The Atomic Energy Commission, which in 1948 offered Blau a promising contract for working at Columbia's laboratories and later at the Brookhaven cyclotron, was the agent which carried out the most extensive production and distribution of isotopes to "friendly nations" for research and medical purposes in the late 1940s. After the end of the Manhattan Project, the AEC also decided that it could use the nuclear piles that had produced plutonium for the bomb as a source of radioisotopes for biomedical research and therapeutic purposes in the local U.S. market. The reactors at Oak Ridge where Rona found employment after she emigrated from Europe could produce, for example, 200 millicuries of carbon-14 in a few weeks for about $10,000, an amazing income for the laboratory.47
52The big machines brought big money and opened opportunities for prestigious research positions. The way the Los Alamos Laboratory, for example, advertised research positions in the mid 1950s leaves no doubt that women were not welcomed as experimenters in this new "big" science: "Excellent opportunities now exist at Los Alamos for qualified men wishing to further their scientific careers . . . The future resides in men with imagination" (emphasis mine).
53At the same time, readers were exposed to subtle images about women's role as the privileged wives who could "live and raise a family" in "an ideal small city" where the laboratory was located.48 In a best-case scenario, women's role in the large laboratories shifted from active experimenters to scanners, calculators, and assistants.49 Coupled with the fact that in the mid-1950s, the proportion of all science and engineering degrees awarded to women remained under 10 percent, the images above—no matter which way we view them—remained unappealing to female scientists.50
54Throughout this book, I have tried to convey a sense of how trafficking materials and gendered experimental practices are tied together in a specific local context, how they can eventually shape an entire laboratory culture, and how the handling of materials constructs gender differences in scientific practice. My concern has been with a history of traces of materials and the study of the people who actually produced, traded, manipulated, consumed, and used them. In the hands of early-twentieth-century physicists, radium evolved into a powerful agent. In the period following the Second World War, as radium lost its power and radioactivity research came to an end, physicists, and especially women physicists, eventually experienced the same fate.
^topNotes
Note 1: Galison, Image and Logic (1997), 454. back
Note 2: Perlmutter, "Marietta Blau's Work After World War II" (unpublished manuscript), 2. back
Note 3: Blau and Dreyfus, "The Multiplier Photo-Tube" (1945). back
Note 4: Karlik, "Eine Lumineszenzmethode" (1933). Karlik presented the modified scintillation counter on February 23, 1933 at a Vienna Academy meeting. During the same meeting, she also presented her work with Rona on the use of the instrument for the study of ranges of alpha-particles emitted from actinium and its products; see Karlik and Rona, "Untersuchungen der Reichweite" (1933). back
Note 5: Blau and Dreyfus, "The Multiplier Photo-Tube" (1945), 246. back
Note 6: Karlik's device was similar to the one that Adolf Krebs developed in 1941. Krebs was a staff member of the Kaiser-Wilhelm Institute for Biophysics in Frankfurt since 1937. In 1947, he became director of the division of Radiobiology of the U.S. Army Medical Research Laboratory at Fort Knox. Krebs, "Ein Demonstrationsversuch" (1941). See also Rheinberger, "Putting Isotopes to Work" (1999), 7; Krebs, "Early History of the Scintillation Counter" (1955). back
Note 7: Blau and Dreyfus, "The Multiplier Photo-Tube" (1945). back
Note 8: Perlmutter, "Marietta Blau's Work," (unpublished manuscript), 2. back
Note 9: Curran and Baker, "Photoelectric Alpha-particle Detector" (1948), 116; Curran and Baker, A photoelectric Alpha-Particle Detector, U.S. Atomic Energy Commission Rpt. MDDC 1296, November 17, 1944, declassified September 23, 1947. See also Galison, Image and Logic (1997), 455; Perlmutter, "Marietta Blau's Work" (unpublished manuscript), 2; Rheinberger, "Putting Isotopes to Work" (1999), 7. back
Note 10: Blau and Dreyfus, "The Multiplier Photo-Tube" (1945), 248. back
Note 11: The Energy Employees Illness Compensation Act of 2000 ("Act" Public Law 106–398) signed by president Bill Clinton in December 7, 2000, established a program to provide compensation to individuals who developed illnesses as a result of their employment in nuclear weapons production. The two corporations that Blau worked for between 1944 and 1948, the International Rare Metals Refinery Inc (listed as Atomic Weapon Employer) and the Canadian Radium and Uranium Corporation, both in Mount Kisco, an hour outside New York, are included in the long list of covered facilities (Department of Energy 6450–010p). The Canadian Radium and Uranium Corp was founded in 1943 and collected radium from airplane industries and watch dials; see Hughes, B. "U.S. Begins Compensating Workers Exposed to Toxic Substances," The Journal News, August 20, 2001. Boris Pregel and his brother Alexander, Russian bourgeois who lived in Paris, came to New York in the early 1940s and established the company as one of the main uranium providers. Alexander was the administrative vice-president and both brothers looked after refugee scientists after World War II. Elisabeth Rona worked for them as well (all the information related to the Canadian Radium and Uranium comes from my personal communication to Vilma Hunt, retired professor of Environmental Health at Harvard School of Public Health. Her information is based on extensive interviews with the Pregels). back
Note 12: Blau and Feuer, "Radioactive Light Sources" (1946). Blau and Feuer constructed a device for using the fluorescent effect of radioactive radiation and especially that of the highly ionizing alpha radiation as a light source. Blau's contributions were based on Karlik's 1933 paper and the use of photocell, this time for transforming the alpha particles into light. The application of the radioactive light sources was enormous. For example, they were used for the standardization of the color of luminous compounds for television purposes. They were also utilized as a source of light for instruments previously painted with luminous compounds dangerously mixed with radioactive material. See, for example, the case of radium dial painters and the use of radium paint in dials of watches and instruments. Rentetzi, "Women Radium Dial Painters" (2004). back
Note 13: Blau and Carlin, "Industrial Applications of Radioactivity" (1948), 82. back
Note 14: News of the University of Miami, 1 April 1960, AUM. back
Note 15: Blau and Smith, "Beta-ray Measurements and Units" (1948), 67. back
Note 16: Marshall and Coltman, "The Photo-Multiplier Radiation Detector" (1947), 528. back
Note 17: Broser and Kallmann, "Über die Anregung" (1947); Broser and Kallamn, "Über den Elementarprozess" (1947). The sensitivity of naphthalene, the first organic and large volume scintillator, made Kallman's counter more efficient than the previous ones. back
Note 18: Rheinberger, "Putting Isotopes to Work" (1999), 8; Perlmutter, "Marietta Blau's Work" (unpublished manuscript). back
Note 19: Perlmutter, "Marietta Blau's Work" (unpublished manuscript). back
Note 20: J. C. Potter to Blau, August 25, 1947, CLP; H.P. Yockey to Blau, September 4, 1947, CLP; Boris Pregel to Blau, November 3, 1947, CLP. back
Note 21: George Pegram to Blau, December 23, 1947, CLP. back
Note 22: Einstein to Goudsmit, January 5, 1954, AEA. back
Note 23: Goudsmit to Einstein, February 11, 1954, AEA. back
Note 24: Rheinberger, "Putting Isotopes to Work" (1999), 7; Galison, Image and Logic (1997), 454–63; Mayneord and Belcher, "Scintillation Counting and its Medical Applications" (1950), 259. back
Note 25: Pringle, "The Scintillation Counter" (1950). back
Note 26: Rheinberger, "Putting Isotopes to Work" (1999), 2. back
Note 27: Medical Radiation Technologists, Web site
http://jobfutures.ca/noc/print/3215.html. back
Note 28: Schrödinger to the Nobel Committee for Physics, January 8, 1950, NARSAS. back
Note 29: Sime, Lise Meitner (1996), 290. Crawford, Sime, and Walker, "A Nobel Tale of Postwar Injustice" (1997). back
Note 30: I would like to thank the archivist of the NARSAS, Maria Asp Romefors, for her help in identifying the members of the Nobel Prize committee. back
Note 31: Report by Lindh on Blau and Wambacher, Uppsala, July 1, 1950, NARSAS. I would like to sincerely thank Anders Stephanson for providing a brief translation of both Lindh's and the Nobel committee's reports from Swedish. back
Note 32: Blau, curriculum vitae, Leopold Halpern Papers. Shapiro, "Tracks of Nuclear Particles" (1941). back
Note 33: Maurice Shapiro interview to Laurie Brown, May 30, 1978, AIP. back
Note 34: Shapiro, "Tracks of Nuclear Particles in Photographic Emulsions" (1941). Powell, "Applications of the Photographic Method" (1943) back
Note 35: Crawford, Elisabeth. "Nobel Population 1901–1950: Anatomy of a Scientific Elite" Physics World, 2001
http://physicsweb.org/articles/world/14/11/7. Crawford, Sime and Walker, "A Nobel Tale" (1997). back
Note 36: Committee report, Nobel Committee for Physics, September 25, 1950. NARSAS. back
Note 37: Galison, Image and Logic (1997), 161–186. back
Note 38: Presentation speech by Axel Lindh, member of the Nobel Committee for Physics
http://nobelprize.org/nobel_prizes/physics/laureates/1950/press.html. back
Note 39: Luke Yuan to Harry Robertson, November 11, 1955, AUM. back
Note 40: Crawford, "Nobel Population" (2001). back
Note 41: Robertson to Blau, October 5, 1955, AUM; Blau to Robertson, November 1, 1955, AUM. Although her salary at the Brookhaven was $7,200, she finally accepted the teaching position at the University of Miami for only $5,500 and became an associate professor under the pressure of applying for a research grant to the National Science Foundation in 1956. See memoradum, Charles Doren Tharp, June 6, 1956. AUM; see Collier, Bert. "Woman Scientist Probes the Atom" Miami Herald, October 5, 1958, AUM. back
Note 42: Hans Thirring had also nominated Blau for a Nobel Prize in 1955, but again she was not successful. Thirring to the Nobel Prize Committee, January 29, 1955, CLP. back
Note 43: E. Ramsauer, Department of State to Morton Miller, May 7, 1957, AUM; Morton Miller to the U.S. Department of State, May 2, 1957, AUM. back
Note 44: Blau to Charles Tharp, March 31, 1960, AUM; Charles Tharp to Blau, April 4, 1960. AUM. back
Note 45: Hans Pettersson's report to the International Education Board, April 1928, AUG. back
Note 46: Augustus Trowbridge, memoradum of conversation with Stefan Meyer and Karl Przibram, March 26, 1925, Box 25/360, RAC. back
Note 47: Krige, "Atoms for Peace, Scientific Internationalism and Scientific Intelligence" (2006); Creager, "Tracing the Politics of Changing Postwar Research Practices" (2002). back
Note 48: Advertisements that appeared in the Journal of American Chemical Society (JACS) (either in the front or the last page of each issue, not numbered) in 1955, 77(21); 1955, 77(23); 1956, 78(3); 1955, 77(21). back
Note 49: See Galison, Image and Logic (1997), 199–200. back
Note 50: LaFollette, "Eyes on the Stars" (1988). back
^top